JBC: What happens to plasmalogens, the phospholipids nobody likes to think about
Alzheimer’s disease patients lose up to 60 percent of a component called plasmalogen from the membranes of the cells in their brains, but we don’t know how or why. In in the Journal of Biological Chemistry, researchers at Washington University in St. Louis provide the first report of an enzyme that breaks down plasmalogens, a breakthrough in understanding the molecular processes that occur during Alzheimer’s and other diseases.
Plasmalogens are particularly abundant in the heart and brain, where they are involved in structuring cell membranes and mediating signals. Plasmalogens are phospholipids defined by a particular chemical bond called a vinyl-ether linkage. Due to the technical difficulties of studying plasmalogens, however, many aspects of their biology are unknown, including how the vinyl-ether bond is broken to break down plasmalogens in cells.
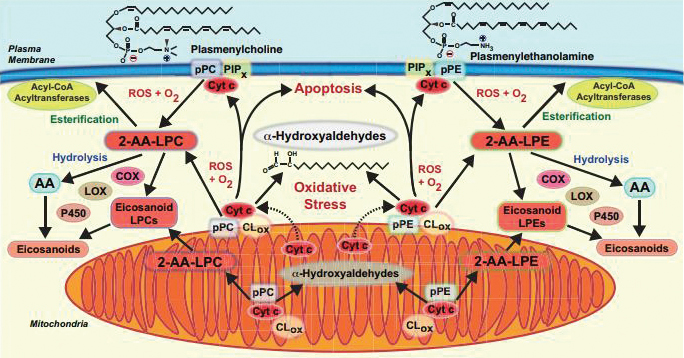 This diagram shows signaling pathways affected by cytochrome c’s degradation of plasmalogen. Courtesy of Richard Gross is the researcher at Washington University who oversaw the new study. “These molecules, plasmalogens, have been swept under the rug because nobody likes to think about them,” Gross said. “(They’re) hard to work with. They’re susceptible to light, they’re stable in only certain solvents, they have a limited lifespan after they’re synthesized unless extreme precautions are taken, and they’re expensive to make and synthesize.”
This diagram shows signaling pathways affected by cytochrome c’s degradation of plasmalogen. Courtesy of Richard Gross is the researcher at Washington University who oversaw the new study. “These molecules, plasmalogens, have been swept under the rug because nobody likes to think about them,” Gross said. “(They’re) hard to work with. They’re susceptible to light, they’re stable in only certain solvents, they have a limited lifespan after they’re synthesized unless extreme precautions are taken, and they’re expensive to make and synthesize.”
In the new study, Gross’ team performed painstaking experiments to find the elusive mechanism by which plasmalogens are enzymatically degraded. Cytochrome c is a protein typically found in mitochondria, where it facilitates electron transport. It can be released into the cell under stressful conditions.
Gross’ team showed that cytochrome c released from the mitochondria can acquire a new function: acting as a peroxidase to catalyze the breakdown of plasmalogens in the cell. Further, the products of this reaction are two different lipid signaling molecules that previously were not known to originate from plasmalogen breakdown.
“That was one thing that surprised us,” Gross said of the signaling products. He said he also was surprised by the ease with which the bond is broken. “The implication is that there is probably a lot of plasmalogen (breakdown) that’s going on in conditions of oxidative stress.”
The results tie in with another observation about the brain cells of Alzheimer’s disease patients, which is that they often have dysfunctional mitochondria and a resultant release of cytochrome c.
Gross is interested in delving deeper into how and why plasmalogen loss occurs in Alzheimer’s patients, particularly those who develop the disease in old age, not due to familial mutations. Gross speculates that as people age, the accumulation of reactive oxygen species leads to cytochrome c release, activation of its peroxidase activity and plasmalogen breakdown in many membranes.
The results also have implications for understanding disorders in the heart and other plasmalogen-rich tissues, integrating studies of mitochondria, cell membranes and cell signaling under stressful conditions.
“This is like a quantum jump into the future,” Gross said.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we鈥檒l send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Mapping fentanyl鈥檚 cellular footprint
Using a new imaging method, researchers at State University of New York at Buffalo traced fentanyl鈥檚 effects inside brain immune cells, revealing how the drug alters lipid droplets, pointing to new paths for addiction diagnostics.

Designing life鈥檚 building blocks with AI
Tanja Kortemme, a professor at the University of California, San Francisco, will discuss her research using computational biology to engineer proteins at the 2026 ASBMB Annual Meeting.

Cholesterol as a novel biomarker for Fragile X syndrome
Researchers in Quebec identified lower levels of a brain cholesterol metabolite, 24-hydroxycholesterol, in patients with fragile X syndrome, a finding that could provide a simple blood-based biomarker for understanding and managing the condition.

How lipid metabolism shapes sperm development
Researchers at Hokkaido University identify the enzyme behind a key lipid in sperm development. The findings reveal how seminolipids shape sperm formation and may inform future diagnostics and treatments for male infertility.

Mass spec method captures proteins in native membranes
Yale scientists developed a mass spec protocol that keeps proteins in their native environment, detects intact protein complexes and tracks drug binding, offering a clearer view of membrane biology.

Laser-assisted cryoEM method preserves protein structure
University of Wisconsin鈥揗adison researchers devised a method that prevents protein compaction during cryoEM prep, restoring natural structure for mass spec studies. The approach could expand high-resolution imaging to more complex protein systems.

.jpg?lang=en-US&width=300&height=300&ext=.jpg)