Crystal building blocks of triglycerides
Francis Crick once said, “If you want to understand function, study structure.” Do you agree? I certainly do, and I would argue that most lipid biologists do too. Just consider the effort to define chemical structures for the many thousands of lipids that exist and the hypotheses about individual lipid function that this structural information has generated.
What has lagged behind is the characterization of the structures of the proteins that modify, transport or interact with these lipids, but times are changing. For example, when I started my postdoc in lab, only a handful of sphingolipid-metabolizing enzymes had been structurally characterized, and these were mainly from bacteria. While many questions remain open (hey, ceramide synthase — we can’t wait to see what you look like!), work from several labs has defined the structures and mechanisms for many human enzymes in sphingolipid metabolism.
A similar revolution appears to be happening with triglycerides. As most of you know, triglycerides serve as a reservoir for energy storage, but when they accumulate excessively, they can cause health problems, including obesity, diabetes and heart disease. Three new structures in particular have caught my attention.
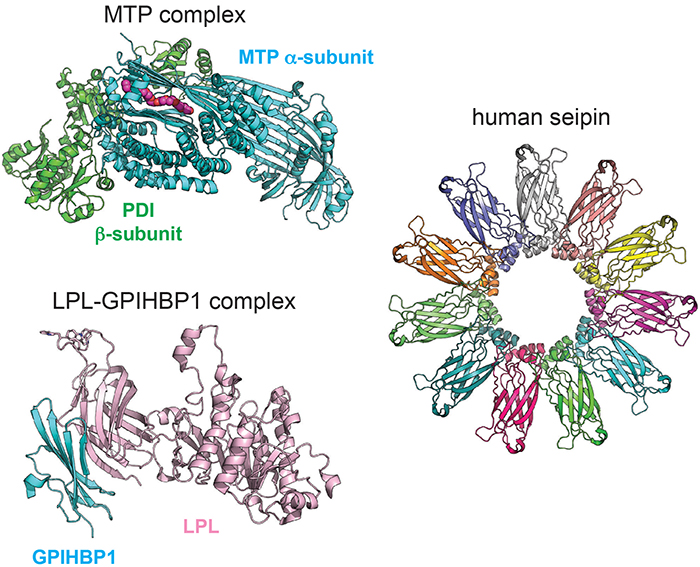 Beautiful structures of proteins involved in triglyceride metabolism and storage.Michael Airola is a crystal structure of microsomal triglyceride transfer protein complex, which transfers neutral lipids into apolipoprotein B-containing lipoproteins. The arduous crystallography required to conduct this work is impressive. The researchers revealed an unexpected lipid-binding cavity and provided insight into disease mutations as well as pharmacological inhibition of this therapeutic target.
Beautiful structures of proteins involved in triglyceride metabolism and storage.Michael Airola is a crystal structure of microsomal triglyceride transfer protein complex, which transfers neutral lipids into apolipoprotein B-containing lipoproteins. The arduous crystallography required to conduct this work is impressive. The researchers revealed an unexpected lipid-binding cavity and provided insight into disease mutations as well as pharmacological inhibition of this therapeutic target.
The second is the crystal structure of lipoprotein lipase, or LPL, the major lipase that clears triglycerides in the blood. and separately determined the LPL structures, overcoming the relative instability of LPL by complexing it with its binding partner glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1. These structures, along with other biochemical data, suggest LPL is active as a monomer, challenging the long-standing paradigm that LPL was only active as a dimer.
The last notable structure is that of seipin, a homo-oligomeric integral membrane protein that is a key player in the formation of cytoplasmic lipid droplets. Two groups ( and ), using cryo-electron microscopy, found that 11 or 12 seipin molecules (dependent on the species) come together to form a ring that spans the endoplasmic reticulum membrane, can bind phosphatidic acid and may stabilize the formation of nascent lipid droplets.
What’s next? Who knows, but I’m darn sure we’re all gonna love it.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Mapping fentanyl’s cellular footprint
Using a new imaging method, researchers at State University of New York at Buffalo traced fentanyl’s effects inside brain immune cells, revealing how the drug alters lipid droplets, pointing to new paths for addiction diagnostics.

Designing life’s building blocks with AI
Tanja Kortemme, a professor at the University of California, San Francisco, will discuss her research using computational biology to engineer proteins at the 2026 ASBMB Annual Meeting.

Cholesterol as a novel biomarker for Fragile X syndrome
Researchers in Quebec identified lower levels of a brain cholesterol metabolite, 24-hydroxycholesterol, in patients with fragile X syndrome, a finding that could provide a simple blood-based biomarker for understanding and managing the condition.

How lipid metabolism shapes sperm development
Researchers at Hokkaido University identify the enzyme behind a key lipid in sperm development. The findings reveal how seminolipids shape sperm formation and may inform future diagnostics and treatments for male infertility.

Mass spec method captures proteins in native membranes
Yale scientists developed a mass spec protocol that keeps proteins in their native environment, detects intact protein complexes and tracks drug binding, offering a clearer view of membrane biology.

Laser-assisted cryoEM method preserves protein structure
University of Wisconsin–Madison researchers devised a method that prevents protein compaction during cryoEM prep, restoring natural structure for mass spec studies. The approach could expand high-resolution imaging to more complex protein systems.

