The many layers of cholesterol regulation
Cholesterol levels in the membranes of animal cells are regulated carefully to remain within narrow limits. Regulation is carried out by a network of proteins that resides in the endoplasmic reticulum, or ER, and controls the two pathways by which cells obtain cholesterol: synthesis and uptake from circulating lipoproteins. The key proteins of this network include a . The sensor is Scap, a polytopic ER membrane protein that binds membrane cholesterol. The transcription factor is a domain of another ER membrane protein called sterol regulatory element-binding protein, or SREBP.
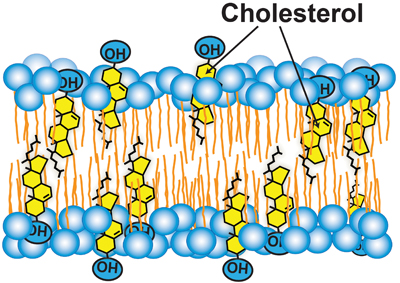 Cholesterol accessibility at the surfaces of membranes rises sharply when its concentration exceeds a threshold, and plays a role in regulating the total cellular level and intracellular distribution of cholesterol. courtesy of Anna Sokolov and Arun Radhakrishnan​
Cholesterol accessibility at the surfaces of membranes rises sharply when its concentration exceeds a threshold, and plays a role in regulating the total cellular level and intracellular distribution of cholesterol. courtesy of Anna Sokolov and Arun Radhakrishnan​
When ER cholesterol is low, Scap initiates a series of molecular events that eventually release SREBP’s transcription factor domain into the cytosol so it can travel to the nucleus to upregulate genes for cholesterol synthesis and uptake. When ER cholesterol rises above a threshold, Scap binds cholesterol and undergoes a conformation change that blocks the processing of SREBPs. Thus, Scap spearheads a feedback mechanism that ensures rapid adjustments to changes in cellular cholesterol levels to ensure cholesterol homeostasis.
However, the cellular distribution of cholesterol poses a significant challenge to this feedback mechanism. Seventy to 90 percent of the cell’s cholesterol is located in the plasma membrane, or PM, whereas Scap is in the ER, which contains only about 1 percent of the cell’s cholesterol. If Scap is to execute its sensing function, the cholesterol-poor ER must be in constant communication with the cholesterol-rich PM so it can be notified promptly of changes in cholesterol levels. Without such a link, Scap would be blind to changes in cellular cholesterol. Indeed, disrupting this link through the use of a toxin that sequesters cholesterol in the PM. In response to this artificial induction, Scap activates SREBPs even though cellular cholesterol has not been depleted.
How are cholesterol levels in ER linked to those in PM? This process requires mechanisms to transport hydrophobic cholesterol across the aqueous cytosol and mechanisms to regulate these transport pathways. Cholesterol transport likely involves a combination of vesicular, nonvesicular and membrane contact site-mediated pathways, and remains poorly understood. We know a little more about how this transport may be regulated. Recent work has used soluble cholesterol-binding toxins to assay the exposure of cholesterol at the surface of purified PMs. revealed that PM cholesterol was sequestered in the membrane bilayer and inaccessible to toxins until it exceeded a threshold concentration of about 35 mole percent of total PM lipids. Above this sharp threshold, PM cholesterol was accessible to bind to toxins. Sharp changes that have been observed for may occur at similar thresholds.
It is tempting to speculate that intracellular cholesterol transport pathways are also sensitive to a sharp change in accessibility of cholesterol on the cytoplasmic leaflet of the PM, allowing for transport to ER to occur only after the PM’s cholesterol needs have been satisfied. How subthreshold levels of cholesterol are sequestered in the PM to prevent interactions with the intracellular transport machinery remains a mystery. We have learned a lot, but there are many more layers of cholesterol regulation yet to be revealed.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Mapping fentanyl’s cellular footprint
Using a new imaging method, researchers at State University of New York at Buffalo traced fentanyl’s effects inside brain immune cells, revealing how the drug alters lipid droplets, pointing to new paths for addiction diagnostics.

Designing life’s building blocks with AI
Tanja Kortemme, a professor at the University of California, San Francisco, will discuss her research using computational biology to engineer proteins at the 2026 ASBMB Annual Meeting.

Cholesterol as a novel biomarker for Fragile X syndrome
Researchers in Quebec identified lower levels of a brain cholesterol metabolite, 24-hydroxycholesterol, in patients with fragile X syndrome, a finding that could provide a simple blood-based biomarker for understanding and managing the condition.

How lipid metabolism shapes sperm development
Researchers at Hokkaido University identify the enzyme behind a key lipid in sperm development. The findings reveal how seminolipids shape sperm formation and may inform future diagnostics and treatments for male infertility.

Mass spec method captures proteins in native membranes
Yale scientists developed a mass spec protocol that keeps proteins in their native environment, detects intact protein complexes and tracks drug binding, offering a clearer view of membrane biology.

Laser-assisted cryoEM method preserves protein structure
University of Wisconsin–Madison researchers devised a method that prevents protein compaction during cryoEM prep, restoring natural structure for mass spec studies. The approach could expand high-resolution imaging to more complex protein systems.

