When it comes to DNA replication, humans and bakerвАЩs yeast are more alike than different
Humans and baker’s yeast have more in common than meets the eye, including an important mechanism that helps ensure DNA is copied correctly, reports a pair of studies published in the journals and .

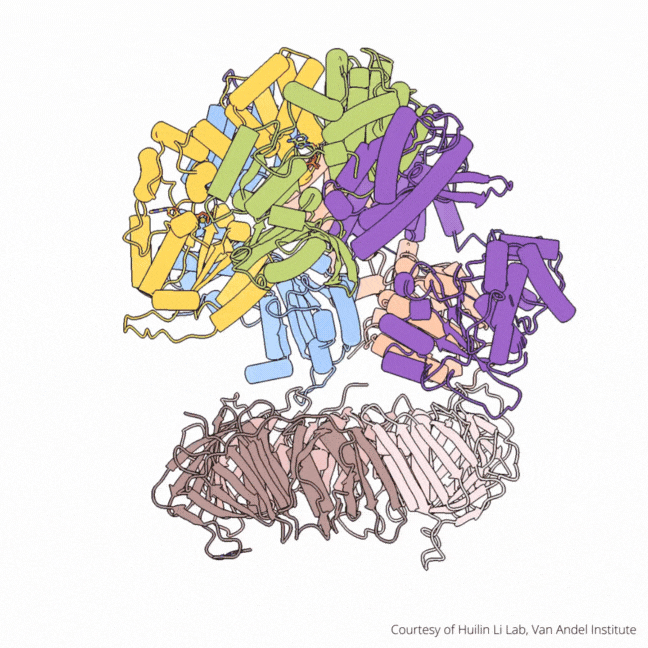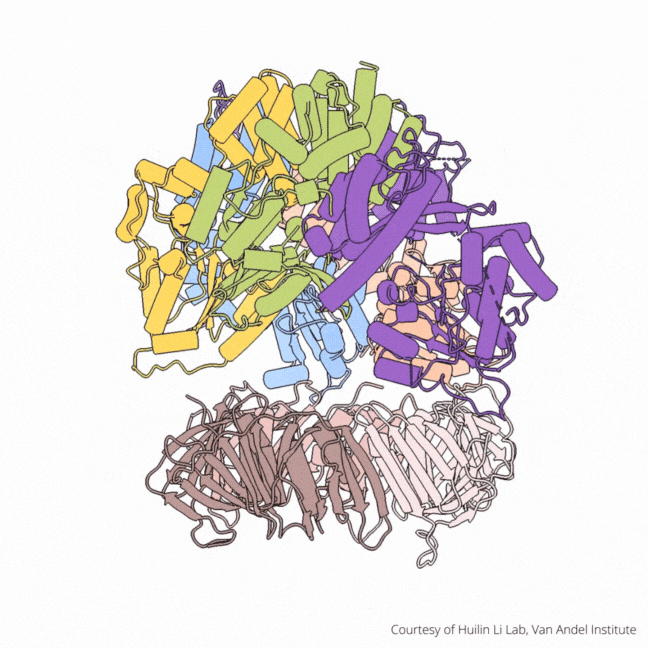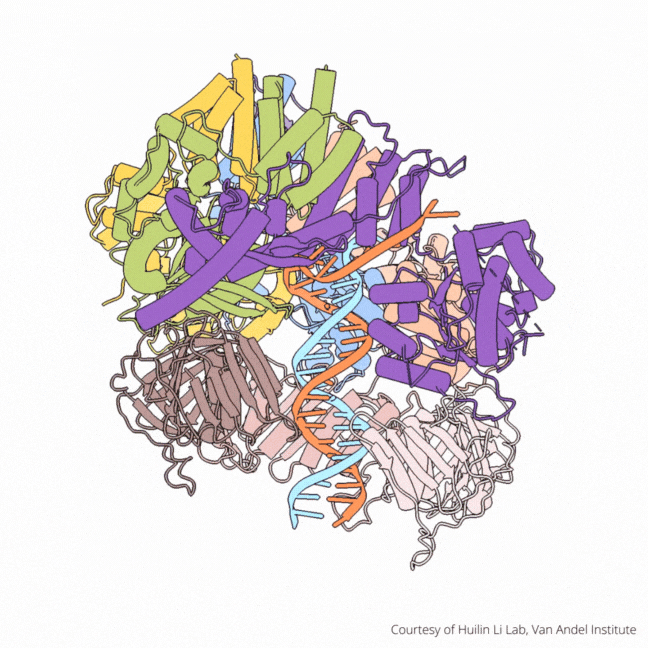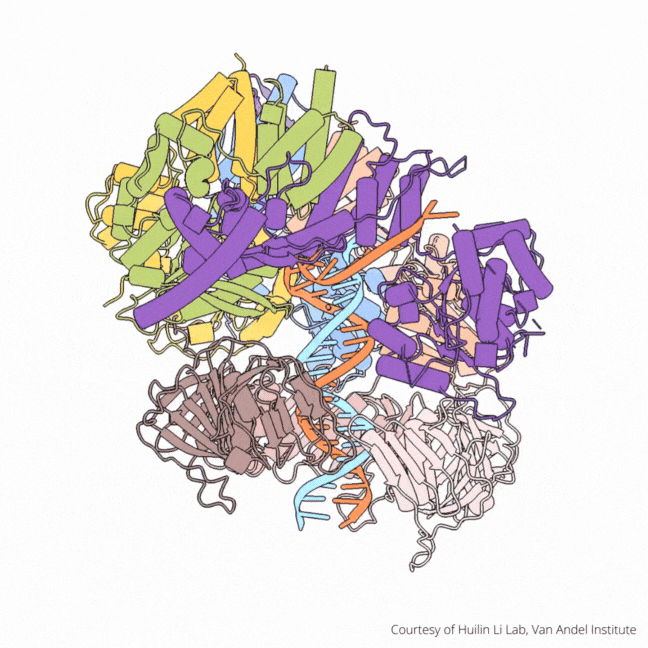
The findings visualize for the first time a molecular complex — called CTF18-RFC in humans and Ctf18-RFC in yeast — that loads a “clamp” onto DNA to keep parts of the replication machinery from falling off the DNA strand.
It is the latest discovery from longtime collaborators of and of t to shed light on the intricate mechanisms that enable the faithful passage of genetic information from generation to generation of cells.
“The accurate copying of DNA is fundamental to the propagation of life,” Li said. “Our findings add key pieces to the puzzle of DNA replication and could improve understanding of DNA replication-related health conditions.”
DNA replication is a tightly controlled process that copies the genetic code, allowing its instructions to be conveyed from one generation of cells to the next. In diseases like cancer, these mechanisms can fail, leading to uncontrolled or faulty replication with devastating consequences.
To date, at least 40 diseases, including many cancers and rare disorders, have been linked to problems with DNA replication.
The process begins by unzipping DNA’s ladder-like structure, resulting in two strands called the leading and lagging strands. A molecular construction crew then assembles the missing halves of the strands, turning a single DNA helix into two. Much of this work falls to enzymes called polymerases, which assemble the building blocks of DNA.
On their own, however, polymerases aren’t good at staying on the DNA strand. They require CTF18-RFC in humans and Ctf18-RFC in yeast to thread a ring-shaped clamp onto the DNA leading strand, and another clamp loader called RFC in both human and yeast to thread the clamp onto the lagging strand. The clamp then closes and signals to the polymerases that they can begin replicating DNA.
Using high-powered cryo-electron microscopes, Li, O’Donnell and their teams revealed previously unknown facets of the leading strand clamp loaders’ structures, including a “hook” that forces the leading strand polymerase to let go of the new DNA strand so it can be recognized by the clamp loader. This distinction represents a key difference between the functions of the leading strand clamp loader (CTF18-RFC) and the lagging strand clamp loader (RFC) and illuminates an important aspect of varying DNA duplication mechanisms on the leading and lagging strands.
Lastly, the study identified shared features between the yeast and human leading strand clamp loaders, which demonstrate an evolutionary link between the two. This finding underscores the value of yeast as powerful yet simple models for studying genetics.
This article is republished from the . Read the original .
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and weвАЩll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Bacteriophage protein could make queso fresco safer
Researchers characterized the structure and function of PlyP100, a bacteriophage protein that shows promise as a food-safe antimicrobial for preventing Listeria monocytogenes growth in fresh cheeses.

Building the blueprint to block HIV
Wesley Sundquist will present his work on the HIV capsid and revolutionary drug, Lenacapavir, at the ASBMB Annual Meeting, March 7вАУ10, in Maryland.

Gut microbes hijack cancer pathway in high-fat diets
Researchers at the Feinstein Institutes for Medical Research found that a high-fat diet increases ammonia-producing bacteria in the gut microbiome of mice, which in turn disrupts TGF-ќ≤ signaling and promotes colorectal cancer.

Mapping fentanylвАЩs cellular footprint
Using a new imaging method, researchers at State University of New York at Buffalo traced fentanylвАЩs effects inside brain immune cells, revealing how the drug alters lipid droplets, pointing to new paths for addiction diagnostics.

Designing lifeвАЩs building blocks with AI
Tanja Kortemme, a professor at the University of California, San Francisco, will discuss her research using computational biology to engineer proteins at the 2026 ASBMB Annual Meeting.

Cholesterol as a novel biomarker for Fragile X syndrome
Researchers in Quebec identified lower levels of a brain cholesterol metabolite, 24-hydroxycholesterol, in patients with fragile X syndrome, a finding that could provide a simple blood-based biomarker for understanding and managing the condition.

